
New viruses aren't always found in exotic locations: for the first time since the measles arose almost 1,000 years ago, a new deadly morbillivirus has emerged. Are scientists facing the grandfather of yet another deadly viral strain, this time from Australia's outback?
By Wilson da Silva
IT CAME FROM NOWHERE. Suddenly, horses were spluttering blood and two men had collapsed. Scientists in bubble suits went into action, scrambling to control the potentially deadly outbreak of a new and unknown virus.
Health authorities were worried. More than a dozen prize racehorses had fallen ill, with temperatures of up to 41˚C. They had difficulty breathing and were spluttering a bloody froth from their mouths and noses. Soon, some began to die a ghastly, wheezing death. Within days, scientists’ worst fears of were realised: both the horse trainer and his stablehand had collapsed, also suffering respiratory problems.
Welcome to the world’s newest Hot Zone. Not the bestseller upon which the Dustin Hoffman film Outbreak was loosely based, but a real life appearance of the most dreaded of microbial beasts: an ‘emerging virus’. A virus that ‘jumps’ from an animal host that – before deforestation and the urban expansion – had little contact with humans. Or it is a new virus, one that was previously harmless to humans, but suddenly mutates and can wreak devastation in its new hosts.
In suburban hinterland outside of Brisbane, bordering on the Australian outback, the world’s newest emerging virus made an appearance last year. At first, the symptoms appeared to match those of African horse virus or of equine influenza, both of which could spread from the stables in Queensland state, Australia’s northeast, and create havoc across the racing industry. But the hospitalisation of two men who had been in contact with the horses raised another, more sinister possibility – one that researchers hoped was wrong.
Samples of horse spleen, lung and blood were sent to the Australian Animal Health Laboratory in Geelong, an imposing concrete structure outside Melbourne that houses one of the world’s most advanced infectious disease centres. Blood samples from the two infected men were also rushed to the infectious diseases ‘hot labs’ of Melbourne’s Fairfield Hospital, and to the Centres for Disease Control and Prevention in Atlanta.
Scientists at the Geelong facility, part of Australia’s Commonwealth Scientific and Industrial Research Organization, received the samples in the early hours of Friday September 23. The canisters were passed through airlocks into the high security inner labs, where diagnostic staff in long, tubular gloves and protected by plastic barriers began a battery of different tests. “We were shooting in the dark,” Paul Selleck, one of the virologists in the diagnostics team, told 21C. “We really had no idea what we were dealing with.”
The researchers worked a blistering pace. The racing industry was already near panic as more of the animals continued to die in the Queensland stables. Pressure from above was increasing.

By late that Friday afternoon, the Geelong researchers had excluded the major viral exotic diseases of horses, including African horse virus and equine influenza. They then worked frantically into the weekend, checking and re-checking their tests. In Queensland, state government veterinarians and health authorities worked on excluding major bacterial diseases and even poison, which might have been the result of foul play. By late Sunday, these had also proved negative.
Meanwhile, the government ordered a quarantine of the area surrounding the Hendra stables in Queensland. All movement of horses in the south of the state were halted. Race meetings were cancelled until further notice. Nearby cattle farmers spoke on talkback radio, saying they feared the disease might infect their animals and grind to a halt the US$2.5 billion a year beef export industry, one of the most disease free in the world. Government veterinarians, also under pressure, combed the stables for more scientific evidence.
While this went on, the health of the trainer, Vic Rail, continued to deteriorate. He was fighting for his life in an intensive care ward of a Brisbane hospital.
There are seven levels of containment at the Geelong facility, each more rigorous than the next. At the highest level, scientists move in ‘bubble suits’, connected to individual air supplies. All liquid and solid waste from the labs is burnt at 120˚C heat of the scrubbers below the labs, including the clothes worn by the researchers when they leave. A whole floor above the labs is taken up with air processing and cleansing facilities, to ensure that not even the smallest of bugs could ever get out. Similarly, anyone contaminated with a deadly organism cannot leave.
All of this weighed on the mind of Dr Peter Hooper as he donned his bubble suit. He had read The Hot Zone – Richard Preston’s account of an outbreak of the deadly Ebola virus from U.S. labs in 1989. The researchers were facing an agent as potentially dangerous, and he was about to handle infected tissue. “That book really affected people’s psychology,” he said. “We knew that we could well be the people in the book. While it was intensely exciting, it was also as scary as it could get.”
Two horses from a local stable were brought into the hermetically sealed rooms of the Geelong facility and left with Hooper. With infected samples from the Queensland horses – minced blood and tissue – Hooper gingerly injected the test horses. Despite the high level of security, he sweated. The holding pens were small. The horses could become agitated, kicking loose his air hose and exposing him to a potentially deadly agent. Thankfully, the horses were well natured.
By the Monday, the microbial culprit had started revealing itself. Cells in most of the cultures prepared three days before had reacted: they were rupturing and coagulating into masses of dead tissue, a cytopathic effect indicating viral action. But this also cause more concern: viruses are usually difficult to grow quickly, yet this virus flourished on a wide variety of cells. This was one virulent bug.
Electron microscopy later revealed that the virus in the cultures had similarities with a large family of viruses called Paramyxoviridae, a large viral family that include measles, canine distemper and a new disease first seen affecting seals in 1994. But it had an array of protrusions like no other paramyxovirus. And no Paramyxoviridae virus is known to cause serious disease in both horses and humans. Not long after this find, scientists in Brisbane also isolated a virus that matched the Geelong discovery.
Further biochemical and serological tests were done to try and isolate which of the three main groups within Paramyxoviridae the virus fitted. But the tests came up blank: the results matched no known Paramyxoviradae. And it was still not clear if the virus was non-lethal just happened to be in the horses, or whether the new bug was the cause of the disease.
On Tuesday, the pressure on the researchers grew. The 49-year-old trainer, who had been in intense care for six days, died in a Brisbane hospital. His lungs had been ravaged by the disease; they had become such a collection of liquefied mush that no samples could be taken. A high security postmortem later took samples instead kidney and spleen, and sent them to the Geelong facility in a special containment canister specially built by health authorities. In Geelong, the samples were inoculated into cell cultures.

“That all of a sudden increased the stakes, because we were possibly dealing with a disease that killed humans as well,” said Selleck. “We still didn’t know if he had in fact died of this virus or not, but we were under considerable pressure to try and resolve the problem. We later tested some serum from Rail against the virus we’d isolated from the dead horses. We found that Vic Rail had antibodies to that virus.”
But not just antibodies: the actual virus too, indicating a virulent organism that could sidestep human defences. “Normally, when the antibody level increases, the virus is cleared from the system, and you are unable to isolate the virus,” Selleck explained. “In this case, the virus was isolated in his kidney, even in the presence of a high antibody level in the blood. That indicates it was able to avoid the immune system.”
The virus now isolated, researchers brought in two other horses and inoculated them with pure virus. If they developed the same symptoms as the Queensland horses and the experimental horses injected on Friday with tissue samples, the researchers would know for certain that they had unmasked the cause.
In the meantime, molecular biologist Dr Allan Gould prepared a series of tests in an attempt to identify the virus’ genetic sequence, using polymerase chain reaction (PCR) techniques. Gould knew that one of the serological tests had detected the merest hint of a reaction to a Rinderpest virus (a so-called ‘morbillivirus’ in the Paramyxoviridae family). But the reaction was so weak that there was a lot of doubt about its accuracy.
So he designed a set of genetic traps – called oligonucleotides or ‘oligos’ in genetic engineering jargon – for the Paramyxoviradae family, and a set that would catch morbilliviruses only. Oligos are strands of synthetic DNA, 20 or so amino acid bases long, which genetic engineers create in any combination. If the oligos bind with a target strand of genetic material, scientists know they have guessed the structure correctly. In this way, oligos act like a sequence of round and square wooden pegs: if the sequence of pegs is correct, all of the right pegs fall into the right holes, and you get a snapshot of the virus’ genetic makeup.
In preparing the two oligos, Gould decided to hedge his bets on the second batch, which was to target morbilliviruses. He picked an area in the generic morbillivirus gene that had some similarities with morbilliviruses in particular, and some similarities with Paramyxoviridae family in general. This wasn’t quite a shot in the dark. But it was an educated guess, a hunch that might be way off the mark. Less than a day later, the results came through.
“None of them came up, except one – and that was against the matrix protein for a morbillivirus,” Gould told 21C. “The matrix protein is the most conserved in morbilliviruses, the least likely to change [as it evolves].”
Switching his focus to morbilliviruses, Gould then prepared oligos recently designed by British researchers which prime specifically for morbilliviruses. But they failed to react. His interest intensified, Gould set about designing his own oligos, zeroing in on the matrix protein of the mystery virus.
He got lucky. But the result was like nothing seen before. “The genome of this new virus was 50 percent different from any other morbillivirus – every second amino acid base was different,” he said. “And that’s in the matrix protein, which is the most conserved. The oligos I’d designed just hung in by their toe-nails. It just worked. If I’d shifted the sequences by just five bases, we would have missed it altogether.”
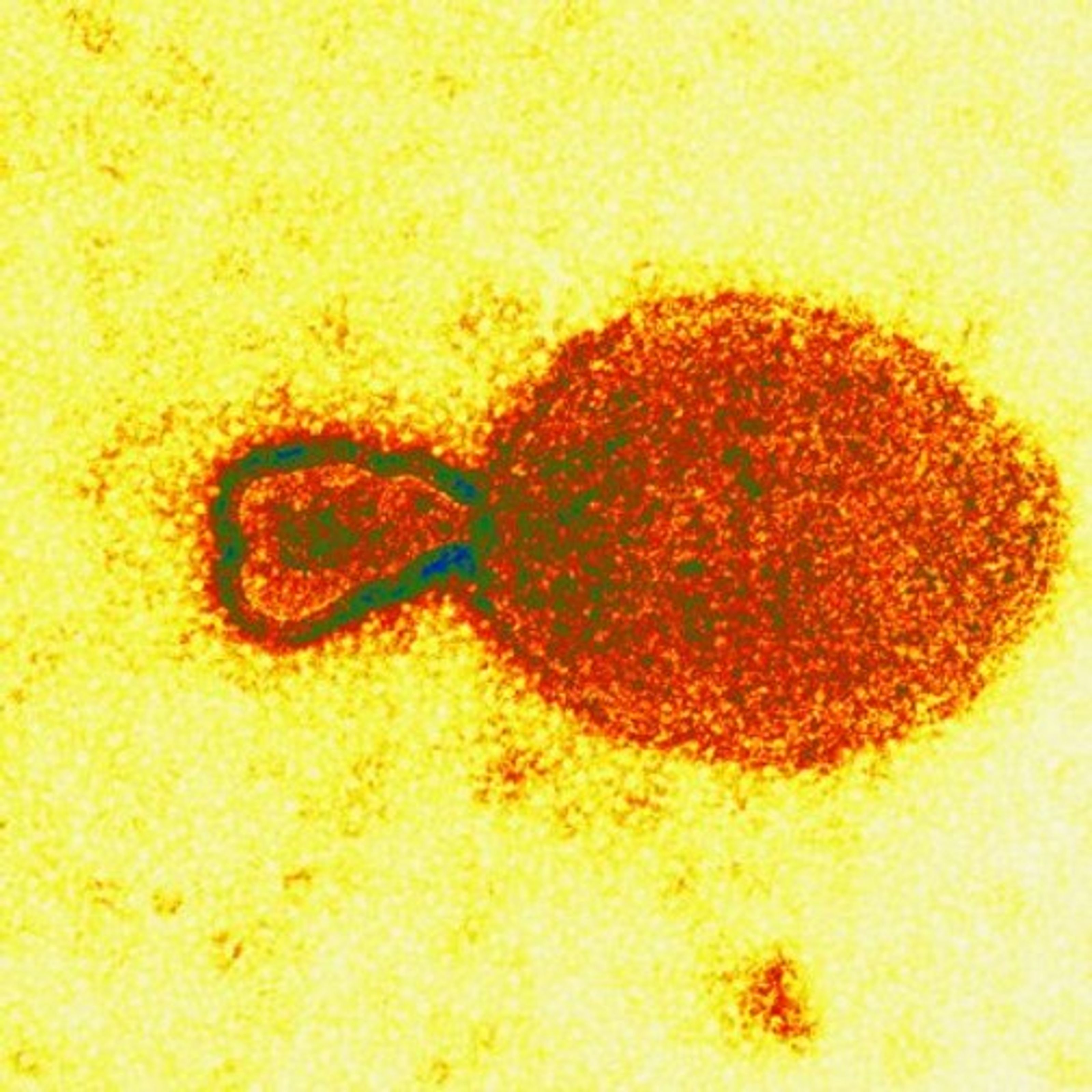
The resulting DNA fragment was sequenced and its structure checked over the Internet with large viral databases in Heidelberg and Cambridge in Europe. The detailed databases confirmed that the Geelong facility had in fact identified a morbillivirus – but one with only the remotest of similarities to known morbilliviruses.
“It’s almost as if this virus is the progenitor of the Paramyxoviridae viruses, as if it’s the grandaddy of them,” Gould said. “At the gene level, we couldn’t find any homology with anything in the databases. When we went to the protein level, yes, then we found similarities with the morbilliviruses.”
Seven days into the process, the Geelong team had identified their likely culprit: the world’s newest emerging virus and the first morbillivirus to infect humans since measles almost 1,000 years ago.
By then, 14 of the 21 racehorses originally infected had died in the Queensland stables. At the Geelong labs, 10 days after being inoculated with the diseased tissue, the first two experimental horses became ill and died within 48 hours, displaying similar symptoms. The other two, injected with pure virus, developed the disease within five days and died soon after. The other infected horses in Queensland recovered slowly, as did the stablehand.
The scientists quickly developed indirect antibody tests for the virus, which were pressed into service. “Every time a horse sneezed anywhere in Australia, we were testing samples the next day,” said Selleck. “We worked every day for about two months, 10 to 11 hour days and through weekends. We had to make sure the virus had not spread.”
Over the next two months, the Geelong facility tested 2,500 horse samples and 150 human samples. All proved negative. Authorities relaxed: the outbreak was contained.
But mystery remained about the origin of the new virus. Research teams have since tracked the disease back to a pregnant mare that was brought to the Hendra stables from a property in nearby Cannon Hill, and died shortly after showing similar symptoms. Traces of the virus were found in the mare. Another horse from the Cannon Hill died at around the same time, but its remains were destroyed before scientists could get to it.
The so-called ‘reservoir’ of the virus remains a mystery, a fact that worries Dr Keith Murray, head of Geelong’s Australian Animal Health Laboratory. “That is quite critical, because if we know the source, we can make some estimate as to how threatening that would be in the future,” he said. “Also, if we know the source, we can when necessary take steps to block re-emergence.”
Murray and his staff suspect the organism resides in a native animal, perhaps a marsupial, since many of these are unique to Australia. It may have happily inhabited an ecological niche in northeastern Australia for millennia, infecting marsupials and causing a mild flu.

Perhaps it only occasionally jumped to humans, and perhaps the odd horse as Europeans settled in the area just over two centuries ago. And now, urban expansion and deforestation may have disturbed the virus, causing it to come into contact with larger populations, making it noticeable. And deadly.
“We know from the gene sequence the it isn’t a recent, single mutational shift. It’s been around for a long time ... and morbilliviruses, so far as it is known, are viruses of mammals,” Murray said.
Seven of the infected horses survived, but Queensland authorities eventually sacrifice them all, in case the virus was capable of re-infecting. Meanwhile, they continue to search for the reservoir species, mindful that – like the recent outbreak of the Ebola virus in Zaire – it could return to kill again.
“Everybody would wish that this was a single incident. But common sense says it could well come back, and we have to be as prepared as we can,” Murray said.
