
The rapid response to COVID-19 by the Australia’s university science sector helped avert an early disaster.
By Wilson da Silva
“WE HAVE something that turned out to be my worst nightmare,” Dr Anthony Fauci, the top infectious disease specialist in the US, told biotechnology executives at a virtual conference in June. “In a period of four months, it has devastated the world.”
At the time, it was six months after the world learned that a new virus — SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus 2) — had jumped to humans in Wuhan, China. More than 7.2 million people were known to be infected globally, and 411,000 had died of the disease it causes, COVID-19.
The numbers have since climbed astronomically, and it could easily have been a calamity for Australia. When the first four travellers from China tested positive on Jan 25 – one in Victoria and three in NSW – Australia ranked 4th in the world, just behind the US. Yet, when Fauci spoke, Australia ranked 67th with 7267 infected and more than 100 deaths, while the US led the world in number of infections and deaths.
Australia’s position remains fragile and it could not have been achieved without an army of highly skilled university researchers, who dropped everything to join the battle.
One such lightning collaboration was a proof-of-concept study to track COVID-19 in raw sewage as a potential early warning system.
In late March, University of Queensland’s (UQ) environmental health scientists Professors Kevin Thomas and Jochen Mueller approached CSIRO Land and Water’s environmental microbiology group to help accelerate the detection of specific gene fragments from complex sewage samples.
Thomas says the open collaboration of the international scientific response to COVID-19 was immediate and immense. “It is a sign that academic collaboration has changed for the better.”
“It’s been just remarkable,” agrees Dr Paul Bertsch, Science Director at CSIRO Land & Water in Brisbane.
“I’ve never seen collaboration at this scale and at this speed. Most collaborations become strong over time, but building relationships is usually done over many years, not weeks.”
Within three weeks, the team — expanded to include Japan’s Hokkaido University, the University of Notre Dame in the US and CSIRO Agriculture & Food — confirmed SARS-CoV-2 could be detected in sewage, finding virus gene fragments in untreated sewage from two wastewater treatment plants in Brisbane that service 600,000 residents.
Changing lanes
The UQ researchers had been prompted by a Chinese study, published in Nature Medicine in late March, that found SARS-CoV-2 in rectal swabs of asymptomatic children who had nevertheless tested negative to nasal swabs.

Thomas’s Queensland Alliance for Environmental Health Sciences group was already monitoring wastewater to determine consumption and exposure to illicit drugs and pharmaceuticals for the Australian Criminal Intelligence Commission. Could they adapt their wastewater epidemiology to find SARS-CoV-2, they wondered?
They could, and the trial has shown that wastewater can be used to estimate how broadly the virus is circulating in the community, especially in those showing mild or no symptoms. “This is a major development that enables surveillance of the spread of the virus through Australian communities,” says Thomas.
Appearing in the journal Science of the Total Environment, it was the first published study of coronavirus surveillance in wastewater in the world. “UQ and that group at CSIRO had never worked together, yet three weeks later they had a peer-reviewed paper,” says Bertsch. “That level of collaboration — not only across Australia, but globally — is really quite amazing around this crisis.”
Protecting healthcare workers
Just as the Brisbane collaboration began, Nigel Curtis, a Professor of Paediatric Infectious Diseases at the University of Melbourne, began a trial of 2,500 healthcare workers to evaluate the immune-boosting properties of an old tuberculosis vaccine, BCG, to protect against COVID-19 or reduce its
severity.
Within a month, he landed a $10 million donation from the Bill and Melinda Gates Foundation to expand his group’s randomised controlled study to 10,000 healthcare staff across Australia, Spain and The Netherlands.
The trial builds on previous research showing that the century-old Bacille Calmette-Guérin vaccine – still given to over 130 million babies worldwide each year – can boost immunity to a virus similar to SARS-CoV-2. The study will “help our researchers show whether BCG vaccination improves ‘innate’ immunity in frontline healthcare workers to buy crucial time to develop and importantly, validate, a specific anti-COVID-19 vaccine,” Curtis says.
The Australian Government, too, has turned to the university sector, creating the Rapid Research Information Forum (RRIF) to provide ministers with an overview of the best scientific thinking on any topic. Led by Australia’s Chief Scientist and facilitated by the academies, it swiftly assembles research experts at universities and research institutes across Australia and New Zealand and delivers a 1,500-word brief within 10 days.
Its partners now hope the RRIF will become a permanent mechanism to provide scientific input into government.
Heeding the call
In January, just as the news of human-to-human transmission of SARS-CoV-2 broke, genome scientist Associate Professor Parwinder Kaur, from the University of Western Australia (UWA), was in Houston, Texas at the founding lab of the DNA Zoo. Kaur is the lead Australian partner of DNA Zoo, a global initiative with more than 60 collaborators in eight countries working to create reference genomes for threatened species across the tree of life.
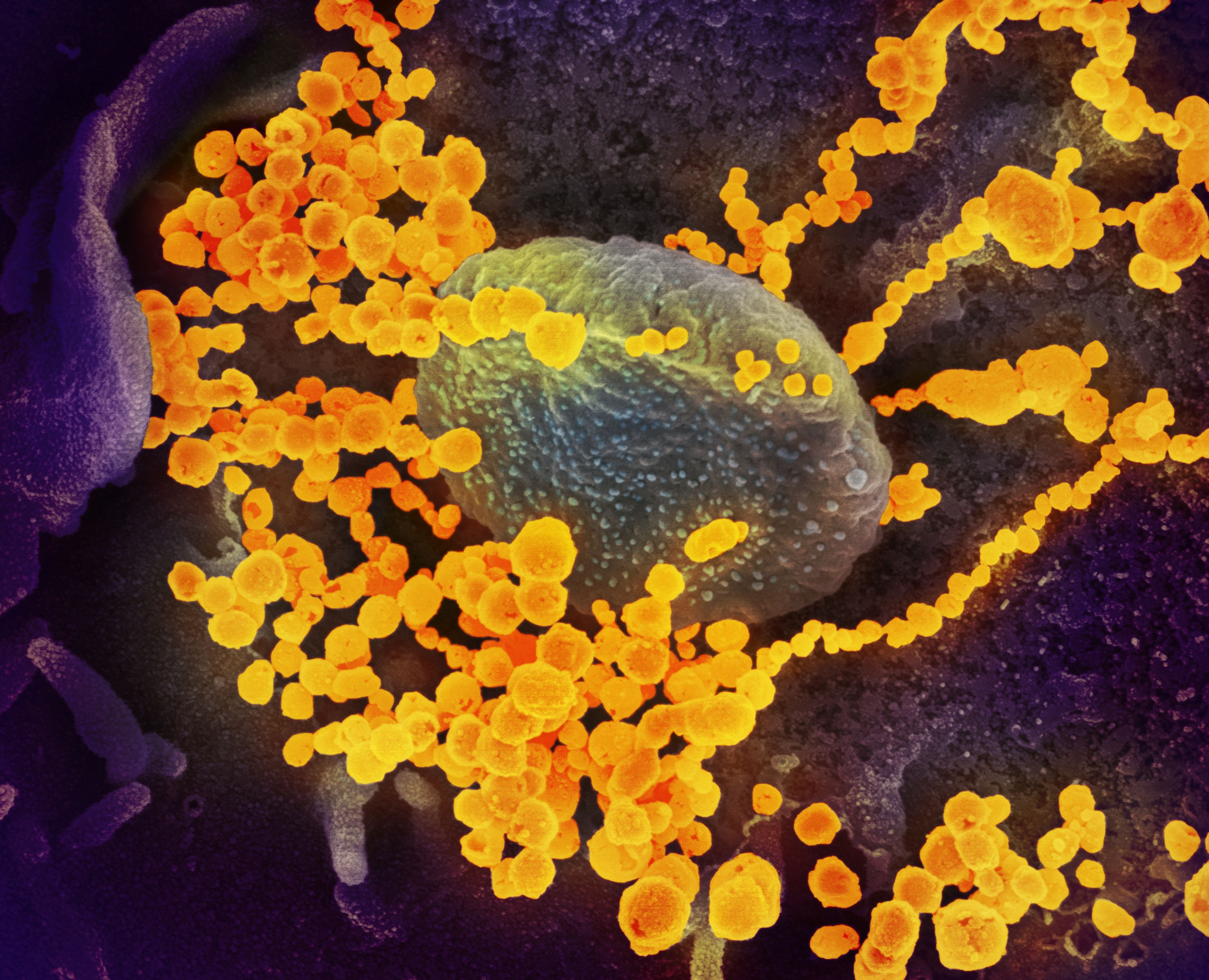
Genomes are assembled using high-resolution 3D models showing the spatial distribution of DNA sequences relative to one another, a technology known as Hi-C that was originally designed to study how genomes fold inside the nucleus.
Importantly, it also allows a very detailed analysis of gene regulation.
As transmission of the virus accelerated, the DNA Zoo team decided to pivot into the effort, decoding the reservoir species of bats and pangolins.
“The team also repurposed the workflow to assemble the whole genome of SARS-CoV-2, as the need of the hour was to understand the epidemiology of the disease,” she adds.
Such a high-resolution model of the genome would not only help understand the virus, but track its mutations. While the Houston lab, led by Dr Aviva Presser Aiden, worked up virus samples, Kaur — now back in Perth — beta tested the data analysis scripts through the nearby Pawsey Supercomputing Centre to build reference genomes.
Cheap, rapid, effective testing
With that data, they were able to develop a diagnostic test, which not only gives a yes/no answer to a patient swab saliva sample, but also the entire virus genome sequence.
In a bioRxiv pre-print paper, Kaur and her DNA Zoo colleagues report that the test accurately detects SARS-CoV-2 in 100 per cent of known samples, and is more than 95 per cent accurate in even small concentrations of virus (84 genome equivalents per millilitre) — better than the limits of detection for almost all diagnostic methods so far approved.
“It is highly sensitive — it detects very low amounts of the virus. And it’s really fast and very cheap,” she says. Using this method, one technician can process 192 samples at a cost of US$30 per patient, including data analysis time.
The partners have applied for emergency use authorisation with both the US Food and Drug Administration and Australia’s Therapeutic Goods Administration. But they’re not done. “We’ve started using this to gather as much epidemiological information as we can, which can be shared with researchers on the front foot of vaccine development,” says Kaur.
“Right now, nobody’s waiting, it’s just an amazing pace. I mean, it’s in none of our KPIs to do this work, and I never thought I would work on a virus genome. But it’s the virus pushing us to do it really, really fast.”

